


Pharma film is a thin-film formed pharmaceutical product that can be taken orally and disintegrates in the mouth; Made by coating and drying the base film solution mixed with active pharmaceutical ingredient and plasticizer, then cut into designated size.
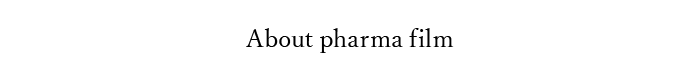
| Responds to needs in an aging society | ・For those who have difficulty in swallowing solids and drinking water, and also who are bedridden |
|---|---|
| Good portability | ・Good to carry as it can be placed in a card case or purse, and can be taken without water ・Can be taken even inside a crowded train or car |
| Fast disintegration | ・Disintegrates on your tongue in 2-8 seconds. |
| Can be printed or designed on top of the film. | ・Print examples: product name, medicine name, content of the API, the efficacy of a medicine and method of ingestion. |
As pharma film can be taken without water,
at time of disaster and in the area where water is limited.
For a person with dysphagia who has difficulty swallowing solid medicine.
For a person confined to bed that it's difficult to swallow water.
| 2004 | Quasi-drug manufacturing authorization |
|---|---|
| 2007 | Drug manufacturing authorization |
| 2010 | Medical equipment manufacturing industry permission |
| 2013 | The second-class drugs manufacturing and marketing permission |
| 2014 | The first-class drugs manufacturing and marketing permissiona |


 Meeting
MeetingThe client discuss with our sales representatives the kind of pharma film product they are hoping to realize.
If necessary, the sales representative will visit the client with our R&D personnel and share the issues of developing the pharma film product.

 In-house conference, study, and schedule the sample making
In-house conference, study, and schedule the sample makingWe would share the information obtained at the meeting with the client to our team.
Study the case and schedule to make samples.
We also study evaluation method after the development stage.

 Formulation study and trial
Formulation study and trialBased on the designed plan, we will perform the Formulation study and conduct the trial.

 Evaluation
EvaluationBased on the results of the trial,
1) Pharmaceutical assessment: Assessment related to the quality of pharma film based on test data of assay and Dissolution test
2) Assessment of samples: evaluation of taste / dissolving condition (property evaluation)
3) Physico-chemical evaluation and analysis of components
4) Physical property evaluation: Perform tests on such physical property as cracking, delaminating and film-thickness test

 Review and evaluation of issues to be solved
Review and evaluation of issues to be solvedBased on the evaluation results, we will take in consideration whether further improvement is necessary.
If improvement is necessary, we review, conduct a retrial, and consult again with the client regarding the plan.